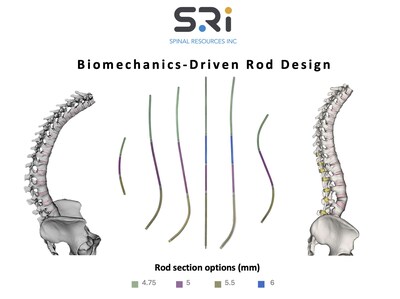
First-of-its-kind Universal Clearance Establishes Maximum Regulatory Access to the Bezier Parametric Curve Spinal Rod System for Patients and Surgeons
PR Newswire
FORT LAUDERDALE, Fla., Feb. 5, 2026
FORT LAUDERDALE, Fla., Feb. 5, 2026 /PRNewswire/ -- The Bezier Parametric Curve Rod System from Spinal Resources, Inc. has received 510(k) clearance for compatibility with any cleared pedicle screw set available on the US market, regardless of manufacturer.
From a patient access perspective, the significance of this clearance cannot be overstated.
By allowing this first-of-its-kind universal clearance, the FDA has taken an important step toward acknowledging the sophistication of both the design and compatibility of SRI's Bezier Parametric Curve Spinal Rod System, as well as enhancing the reach and adoption of truly innovative independent technologies for spine surgeons seeking options for their patients.
Dr. Saman Shabani, MD, the Director of Spinal Oncology Surgery, Adult Spinal Deformity Surgery and the Spine Fellowship Program at the Medical College of Wisconsin, had this to say about the clearance, "From a surgeon perspective, the universal clearance for the Bezier Rod system is ground-breaking because it gives me clinical liberty to achieve the best possible outcomes with the best possible technology without being restricted to a single supplier or device manufacturer construct."
Typically, a spinal fixation rod is cleared for use with, and only with, pedicle screws from the same manufacturer. In addition, hospital systems and group purchasing organizations often restrict the number of vendors – and therefore the technology selections – available to surgeons in their facilities. The result is that surgeon choice and patient benefit are limited to a subset of the technologies available in the overall market.
The combined effect of these two realities is that it limits the opportunity for the surgeon and patient communities to be made aware of the broadest possible technology options and associated clinical benefits. This reality is very frustrating to surgeons who do not want their clinical decisions impacted by product availability.
Bernie Bedor, President and Chief Executive Officer, Spinal Resources, Inc. notes, "We are thrilled to have achieved this universal clearance for our Bezier Rod platform. SRI has taken another step towards our stated goal of addressing persistent challenges in spinal disease by developing the uniquely differentiated and biomechanically superior Bezier Rod system and successfully advocating for broad surgeon and patient access."
About Spinal Resources Inc.
Spinal Resources Inc.® is a Ft. Lauderdale, Florida based spinal medical device company that supports cost-effective patient care with innovative mechanical and bio-mechanical products to alleviate pain, shorten recovery time, restore health, and extend quality of life.
https://spinalresourcesinc.com
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/first-of-its-kind-universal-clearance-establishes-maximum-regulatory-access-to-the-bezier-parametric-curve-spinal-rod-system-for-patients-and-surgeons-302679616.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/first-of-its-kind-universal-clearance-establishes-maximum-regulatory-access-to-the-bezier-parametric-curve-spinal-rod-system-for-patients-and-surgeons-302679616.html
SOURCE Spinal Resources Inc.